Who are the experts. We review their content and use your feedback to keep the quality high.

Covalent Bond Covalent Bonding Chemical Bond Water Molecule
It must be proved wrong or right based on data collected.

. Distinction between elements and compounds. Which is not a type of chemical bonding. See the answer See the answer done loading.
Which types of chemical bonds are formed between atoms of the same molecule. CBonds create new elements. Conduct electricity in molten form or in a solution.
An hypothesis should not be invalid. The electron sea theory accounts for all the following characteristics of metals except. They are then attracted to each other resulting in an Ionic Bond.
Which of the following is not produced through chemical bonding. The measure of an atoms ability to attract electrons to itself when in a chemical bond is called. Here an atom loses an electron which is in turn gained by another atom.
DChemical bonds sustain life. Which of the following is NOT a type of chemical bond. Which of the following is not one of the chemical bonds.
Hypothesis should be clear and not vague. B Polar covalent bond. Due to the mutual sharing of electrons they.
View the full answer. An orderly arrangement of ions in a 3 dimensional pattern within a compound is called. Ionic bonds because the calculation requires the charges of the ions which covalent bonds dont have Collins law The energy between two ions equals the product of the two charges which are represented by capital Qs divided by the distance or radius between the.
Which one of the following properties does not belong to a covalent substance. Experts are tested by Chegg as specialists in their subject area. Jarvis Describe Ionic Bonding.
Three types of chemical bonds are important in human physiology because they hold together substances that are used by the body for critical aspects of homeostasis signaling and energy production to name just a few important processes. These are ionic bonds covalent bonds and hydrogen bonds. 2Which of the statement is false.
Answer - hydrogen bond. Chemical bonds include covalent polar covalent and ionic bonds. Which among the following chemical bond were described by Kossel and Lewis.
Compound names element names number and ratio of atoms Chemical bonds between atoms involve electrons Ionic Bonds. Start studying Identifying Chemical Bonds. Which among the following is not a property of Ionic bond.
Hypothesis must be capable of being tested or verified. Learn vocabulary terms and more with flashcards games and other study tools. In plasma a typical body fluid protein floating around would be considered to be which of the following.
To determine the chemical formulas of ionic compounds the following two conditions must be satisfied. Ionic bonding is a type of chemical bonding which involves a transfer of electrons from one atom or molecule to another. A Covalent B Metallic C All of the answers are correct D Ionic.
ABonds are used to make new substances. The higher the charge the stronger the bond between them. Which chemical bonds are the strongest.
20 Questions Show answers. Which among the following formation is not an example of Covalent bond. Hydrogen and carbon are not bonded while in water there is a single bond between each hydrogen and oxygen.
Which among the following chemical bond were described by Kossel and Lewis. The following two requirements must be met in order to derive the chemical formulae of ionic compounds. Cohesive forces between the atoms depend on the charge on the atoms.
A Two answers are correct B Electrons are given away C Electrons are accepted D electrons are shared. Types of chemical bonds including covalent ionic and hydrogen bonds and London dispersion. D Ionic and Covalent bond.
Result from electron transfer. The luster or shine seen on most. Hypothesis must be specific to the research topic and not.
This problem has been solved. 2 See answers Advertisement Advertisement hayleecox135 hayleecox135. The covalent link is the most powerful chemical bond.
Each ion must obey the octet rule for. When one atom gives up an electron to another atom they both become charged one positive one negative. The valency of an element depends upon.
SYI1 EU SYI1B LO SYI1B1 EK Chemical bonds hold molecules together and create temporary connections that are essential to life. BBuilding and breaking bonds are part of the energy cycle. Chemical bonds are forces that hold atoms together to make compounds or molecules.
Bolivianouft and 3 more users found this answer helpful. Click on the box which contains physical properties of an element which can be examined. Atoms with relatively similar electronegativities share electrons between them and are connected by covalent bonds.
A solute specifically both a colloid and an electrolyte. Which of the following is NOT a reason why chemical bonds are important. This pictures shows examples of chemical bonding using Lewis dot notation.
State whether the given statement is true or false Ionic bonds are non-directional. Hydrogen Bond B. For optimum stability the cation and anion should obey the octet rule.
Same elements may form different compounds Reading chemical formulas. Both Ionic and Covalent bond arise from the tendency of atoms to attain stable configuration of electrons. Which of the following is NOT a chemical bond.
Chemical bonding begins with atoms it does not produce them. Anions are negatively charged. The number of electrons gained lost or shared by an atom in a chemical bond is also known as.
CovalentLewis electron dot structure cations anions positivelose low highcovalent polar covalent nonpolar metallic bond. High melting point and a brittle solid. Atoms adipose fat tissue cells the heart.
These types of bonds in chemical bonding are formed from the loss gain or sharing of electrons between two atomsmolecules. The electromagnetic attraction betwe. For an hypothesis being termed as a valid one it should have the following criteria.

Image Result For Polar Vs Nonpolar Molecules Covalent Bonding Chemical Bond Study Chemistry
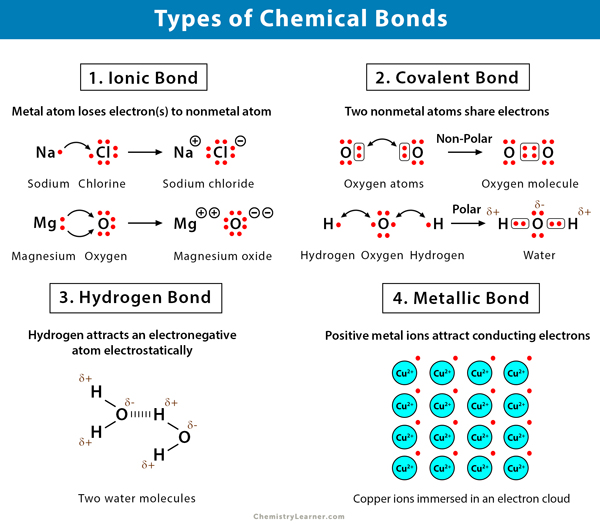
Chemical Bonds Definition Types And Examples

Examples Of Polar And Nonpolar Molecules Chemical Bond Covalent Bonding Chemistry

Coordinate Covalent Bond Definition And Examples Covalent Bonding Chemical Bond Bond
0 Comments